The use of complexants and chelates in micronutrient fertilization
Metal micronutrient deficiencies can be effectively treated with the use of synthetic chelates.
Chelates




Figure 1: Chemical structure of the racemic and meso geometric isomers of Fe-o, oEDDHA and the positional Fe-o, pEDDHA. Note the protection of the central ferric ion, surrounded by 6 donor groups in the oxyda and five in the o, pEDDHA.
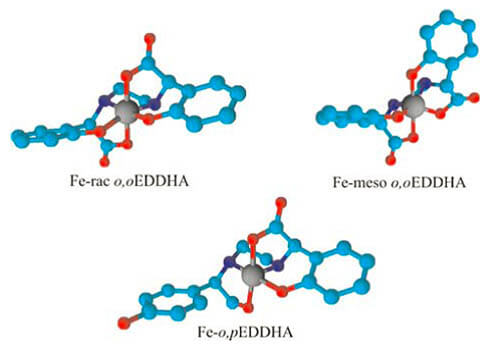
A as of place is very important to take into account the wealth in the isomers and not in the data of soluble Fe, since if it is not chelated it will precipitate and will not be used by the plants (Hernández-Apalolaza et al., 2000; García-Marco & Lucena, 2009). EDDHMA- ethylenediamine acid-N,N’-di(orthohydroxymethylphenylacetic), EDDHSA- ethylenediamine acid-N,N’- di[(2-hydroxy-5-sulfophenylacetic acid] y EDDCHA- ethylenediamino-N, N ‘- [(5-carboxy-2-hydroxyphenyl) acetic] also form high stability ferric chelates. The last two are the most soluble so they can be used in liquid fertilizers (Lucena et al., 2004). HBED – acid N,N’-bis (2-hydroxybenzyl) ethylenediamino-N, N’-diacetic and DCHA- 2- (2 – ((2-hydroxybenzyl) amino) ethylamino) – 2-(2-hidroxiphenil) are two new solutions under study. For non-sensitive cultures, or non-calcareous substrates and with frequent addition of chelate, it is possible to use chelants of lower stability for Fe such as EDTA- etlylenediamine tetraacetic acid, HEEDTA- N- (2-hydroxyethyl) ethylenediaminetriacetic acid and DTPA- diethylenetriaminepentaacetic acid. Recently, the use of IDHA – imidodisuccinic acid as biodegradable chelating agent (Lucena et al., 2008). The most commonly used chelates for the micronutrients Mn, Zn and Cu are DTPA, EDTA, HEEDTA (Lindsay & Sommers 1997) and also IDHA (Lucena et al., 2008). Although its effectiveness in acid soils conditions is high, however none is a completely satisfactory solution, especially for the Mn, if the soil limestone content is very high.
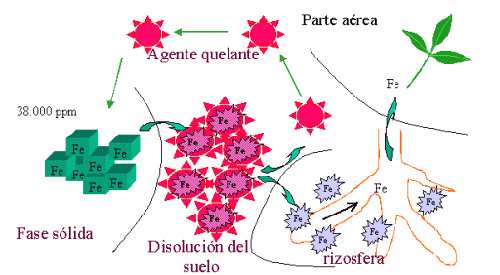
Figure 2: Schematic of the action of the chelating agent
Conclusions
The efficacy of chelates is sufficiently proven, although it must be taken into account that the products that are suitable for each agronomic condition and element must be used at the correct dosage and with products that have a proven commercial quality.More effort is needed in the understanding and evaluation of both the products and their mode of action.
Bibliographic References
Chen Y & Barak P (1982) Iron nutrition in calcareous soils. Advances in Agronomy, 35:217-240.
Álvarez-Fernández A, García-Marco S & Lucena JJ (2005) Evaluation of synthetic iron(III)-chelates (EDDHA/Fe 3+, EDDHMA/Fe3+ and EDDHSA/Fe3+) to correct iron chlorosis. European Journal of Agronomy, 22: 119-130.
García-Marco S, Martínez N, Yunta F, Hernández-Apaolaza L. & Lucena JJ (2006a) Effectiveness of ethylenediamine- N- (o-hydroxyphenylacetic)- N’-(p-hydroxyphenylacetic) acid (o,p-EDDHA) to supply iron to plants. Plant and Soil, 279: 31-40.
García-Marco S, Torreblanca A & Lucena JJ (2006b) Chromatographic determination of Fe chelated by ethylenediamineN(o-hydroxyphenylacetic)- N’(p-hydroxyphenylacetic) acid in commercial EDDHA/Fe3+ fertilizers. Journal of Agricultural and Food Chemistry, 54: 1380-1386.
García-Marco S & Lucena JJ (2009) Mercado español de quelatos férricos sintéticos: evolución de su calidad. Phytoma, 205: 32-36. Garcia-Mina J, Cantera RG & Zamarreño A (2003) Interaction of different iron chelates with an alkaline and calcareous soil: a complementary methodology to evaluate the performance of iron compounds in the correction of iron chlorosis. Journal of Plant Nutrition, 26:1943-1954.
Hernández-Apaolaza L, Álvarez-Fernández A & Lucena JJ (2000) Chromatographic determination of commercial Fe(III)- chelates. Journal of Plant Nutrition, 23: 2035-2045.
Lindsay WL (1979) Chemical Equilibria in Soils. New York, J Willey & Sons. 449 p. Lindsay WL & Sommers LE (1997) Complexation of metals by synthetic chelating agents. In DC Adriano Ed. Biogeochemistry of Trace Metals. Northwood, Science Reviewers. Pp. 283-303.
Lucena JJ (2000) Effect of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. Journal of Plant Nutrition, 23: 1591-1606.
Lucena JJ (2004) Quelatos de hierro conteniendo o,pEDDHA. Estudios sobre su eficacia. Phytoma, 163: 26-32. Lucena JJ (2006) Iron Fertilizers in Correcting Iron Deficiencies in Plants. In: Barton LL & Abadía J (Eds.) Iron nutrition in plants and rhizospheric microorganism. Dordrecht, SpringerVerlag Academic Pubs. Pp. 103-127.
Lucena JJ, Sentís JA, Villén M, Lao T & Pérez-Sáez, M (2008) IDHA Chelates as a micronutrient source for green bean and tomato in fertigation and hydroponics. Agronomy Journal, 100: 813–818.
Lucena JJ, García-Marco S, Yunta F, Hernández-Apaolaza L & Navarro-Rodríguez T (2004) Theoretical modelization and reactivity of the iron chelates in Agronomic conditions. In: J.M. VanBriesen and B. Nowack (Eds.) Biogeochemistry of chelating agents. New York , American Chemical Society. Pp. 348-365.
