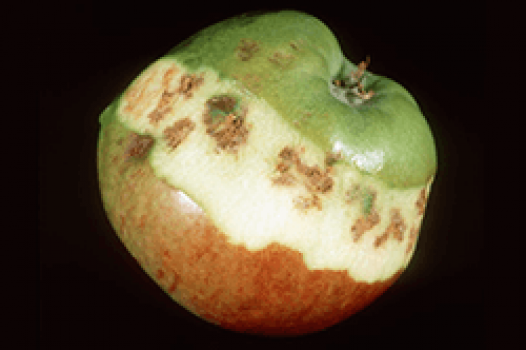
Calcium plays a fundamental role in the formation of the membrane of the cell wall and in its plasticity. This increases normal cell division while maintaining cell integrity and membrane permeability. Calcium also acts as an activator of several enzymatic systems in the synthesis of protein and in the transfer of carbohydrate. Calcium is mixed with anions including organic acids, sulfates and phosphates. It also acts as a detoxifying agent as it neutralizes organic acids in plants. Calcium indirectly helps to increase crop production by reducing the acidity in the soil when it is limestone.
Calcium Deficiency
The importance of microelements in the production of fruit trees and vines of high quality and condition
MANGANESO IN THE PLANTS

Mn Deficiency in Palto

Mn Deficiency in Citrus

Mn Deficiency in Vid
Manganese deficiency
General information


BIBLIOGRAPHIC REFERENCES
- Garcia, E. 2013. Deficiencies of Iron and Manganese in Bean Leaves (Phaseolus vulgaris L.) identified by textural analysis, color of digital images and artificial neural networks. Institute of teaching and research in agricultural sciences. Mexico.
- Gaspar, L. Fertilization of vine cultivation. Agro-strategies consultants.
- INIA Chile. Toxicity of manganese in blueberry plants in the north.
- Sierra, C. 1998. Fertilization of the Palto. INIA Intihuasi.
- INTA Argentina. Manual for orange and tangerine producers.
- Plant nutrition, the role of essential nutrients part II, Micronutrients. Institute for Technological Innovation in Agriculture. Mexico.
- International Plant Nutritiun Institute (IPNI). Know the deficiency of manganese ipni.net/ppiweb/iamex.nsf/$webindex/F9CC2B73823D9E1F06256AD1005E1257/$file/Conozca+la+deficiencia+de+manganeso.pdf
- Schulte, E. E. and Kelling, K A. 1999. Soil and applied manganese. Publication A2526. Wisconsin county Extension office. University of Wisconsin. WI, USA.
- Nutrition and Plant Physiology. Continuing training program. 2016
- National University of Madrid (UNM). Relevant aspects of the Cinc in the plant. Zinc
The importance of microelements in the production of fruit trees and vines of high quality and condition
ZINC IN THE PLANTS
This nutrient accumulates in the roots, through which it is actively absorbed as a divalent cation (Zn2+), and can also be absorbed as a monovalent cation (ZnOH+) to be distributed in the plant and fulfill a series of processes7 y 8. The absorption of Zinc increases with the presence of arbuscular mycorrhizae, and is reduced drastically in low temperatures and by antagonism with other elements7.
If the concentrations of Zn increase above the tolerance threshold, toxicity effects, including chlorosis and reduced plant growth, become evident; inhibition of CO fixation2, the transport of the carbohydrates in the phloem and the alteration of the permeability of the cellular membrane1.
It is necessary to consider a correct fertilization plan according to previous analyzes to optimize the resources and to project an efficient yield of the crop. Zinc sulfate (ZnSO4) is a widely used source of Zn, whose composition is 36%10. In the cultivation of avocado (Hass variety) the appropriate range of Zn in leaves is 30 – 150 ppm10, in blueberry the appropriate values range from 8-30 mg / kg5, while in citrus the optimum values are 25 – 100 ppm6 and in vine they are of 26 – 40 ppm4. In addition, in the case of vine, the optimal values of Zn in the berries when performing an analysis of 100g of dry matter is 0.04-0.08mg.

Deficiency of Zn in Palto

Deficiency of Zn in Citricos

Deficiency of Zn in vid
Deficiency of Zinc
BIBLIOGRAPHIC REFERENCES
- Efroymson, R.A., M.E. Will, G.W. Suter y A.C. Wooten. 1997. Toxicological benchmarks for screening contaminants of potential concern for effects on terrestrial plants. Department of Energy, Office of Environmental Management Activities at the East Tennessee Technology Park. 123 p.
- Fancelli, AL. 2006. Micronutrients in the physiology of plants. Pp 11-27. En: M Vázquez (ed). Micronutrients in agriculture. Argentine Association of Soil Science. Buenos Aires, Argentina.207pp
- Flores, M.; Anchondo, M.y E. Sanchez. 2009. Acidification in band, winter tillage and Zinc in walnut pecan. Chihuahua, Mexico.
- Gaspar, L. Fertilization of vine cultivation. Agro-strategies consultants.
- Hirzel, J. blueberry Fertilization. INIA Quilamapu. Chile.
- INTA, Manual for Orange and Mandarin Producers. Argentina.
- Plant nutrition, the role of essential nutrients part II, Micronutrients. Institute for Technological Innovation in Agriculture. Mexico.
- Perea, E.; Ojeda, D.; Hernandez, O.; Escudero, D.; Martinez, J. y G. López. 2010. Zinc as a promoter of growth and fructification in walnut pecan. Technoscience chihuahua Vol IV N° 2.
- Salazar, S.; Cossio, L. y L. González. 2008. Correction of chronic deficiency of Zinc in Hass avocado. Rev. Chapingo Ser. Hortic. Vol N° 2. Mexico.
- Sierra, C. 2003. Fertilization of crops and fruit trees in the north. INIA, Ministry of Agriculture of Chile.
- Sierra, C. 1998. Fertilization of the Palto. INIA Intihuasi.
- National University of Madrid. Relevant aspects of Zinc in the plant.
uam.es/docencia/museovir/web/Museovirtual/fundamentos/nutricion%20mineral/micro/Zinc.htm. - Wood, B. 2007. Correction of Zinc deficiency in pecan by soil banding. Dep. of Agriculture. HortScience 42: 1554-1558.
Iron in the plants

Deficiency of Fe in Palto

Deficiency of Fe in Citric

Deficiency of Fe in vid
Deficiency of iron

BIBLIOGRAPHIC REFERENCES
- Gaspar, L. Fertilization of vine cultivation. Agro-strategies consultants.
- Know the deficiency of Iron. International Plant Nutritiun Institute. ipni.net
- Iron chlorosis in crops. Institute for Technological Innovation in Agriculture. Mexico.
- Ferreyra R. y Ruiz, R. 2008. Feral Chlorosis of the Palto and irrigation management. INIA Tierra Adentro. INIA La Platina – INIA La Cruz. Chile.
- Hirzel, J. blueberry Fertilization. INIA Quilamapu. Chile.
- Sierra, C. 2017. An intense relationship: El Hierro, the soil and the plant. El Mercurio Journal, Santiago de Chile. Chile.
- Sierra, C. 2003. Fertilization of crops and fruit trees in the north. INIA, Ministry of Agriculture of Chile.
- Sierra, C. 1998. Fertilization of the Palto. INIA Intihuasi.
- Nutrition and Plant Physiology. Continuous training program. 2016
COPPER IN THE PLANTS
Optimal Cu values in leaf analysis are: 5-15 ppm in Palto Hass and fort7, 4-20 mg/kg in blueberry8, 18-34 ppm on vid9, and 6-14 ppm in citrus10. A correct use will allow the success in the production.

Deficiency of CU in Palto

Deficiency of CU in Citric
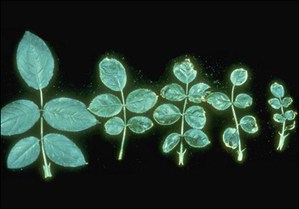
Deficiency of CU in Rosal
BIBLIOGRAPHIC REFERENCES
- International Plant Nutritiun Institute (IPNI). Know Copper Deficiency. ipni.net
- Sierra, C. 2016. A look at the relationship between copper, soil and plants.
- Synergisms and antagonisms between nutrients. Institute for Technological Innovation in Agriculture. Mexico. Tecsup. Nutrition and Plant Physiology. Continuing training program. 2016
- León, J. y G. Sepulveda. 2012. The oxidation damage caused by copper and the antioxidant response of plants. Inter science Vol. 37, N° 11, pp 805-811. Venezuela.
- Nutrition and Plant Physiology. Continuous training program. 2016
- Kirkby, E y Volker, R Micronutrients in Plant Physiology: Functions, Absorption and Mobility. International Plant Nutritiun Institute(IPNI)
- Sierra, C. 1998. Fertilization of the Palto. INIA Intihuasi.
- Hirzel, J. blueberry Fertilization. INIA Quilamapu. Chile.
- Gaspar, L. Fertilization of vine cultivation. Consulting Agro-Strategies.
- INTA, Manual for Orange and Mandarin Producers. Argentina.